EU MDR and the United Kingdom.
29/6/2021
COVID-19 Survey by the EU Commission.
22/6/2021
17/6/2021
The future of Wearable Devices.
15/6/2021
Cambridge Academy of Therapeutic Sciences announces seminar with Med-Di-Dia.
9/6/2021
EU MDR - what’s happening with Switzerland?
8/6/2021
MedTech Europe warns of ongoing regulatory issues
3/6/2021
1/6/2021
The US FDA releases draft guidelines for Medical Devices
27/5/2021
EU plans to enforce additional regulations on MedTech AI products, other 'high-risk' systems.
25/5/2021
Report from Deloitte Spotlights the Trend for Virtual Care
20/5/2021
Main changes introduced by the MDR for Class I Device Manufacturers
18/5/2021
UDI Helpdesk by the EU Commission!
17/5/2021
Synergies of HealthCare and Technology are leading the way!
13/5/2021
Medical Devices are Trending! Here is your Trend Guide
11/5/2021
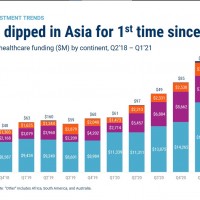
A dip in Asian Healthcare Funding – Opportunity for the EU
6/5/2021
New MDR and IVDR leave Manufacturers in Doubt as guidelines issued for Northern Ireland
4/5/2021
#ScalingOurLegacy - GTC Celebrates 25 + years of Service
29/4/2021
MDCG Recent Guidance on MDR, IVDR
28/4/2021
What are the responsibilities of the EU Authorised Representative?
11/9/2020