If Google Can, So Can You: Mastering the Art of Medical Device Certification

Strategic choices in certification pathways provide valuable insights for any startup in the wellness industry looking to make a global impact.
18.3.2025
European Medical Device Nomenclature (EMDN): A Key to EU Compliance

This comprehensive system is crucial for manufacturers who are navigating the complexities of EU regulations and EUDAMED
28.1.2025
The Human Touch in Regulatory and Quality Compliance: Why Consultants Still Matter in the AI Era

Why not rely solely on AI for creating technical documents, implementing ISO standards, or managing regulatory and quality compliance?
23.1.2025
Mastering TPLC for Medical Softwares
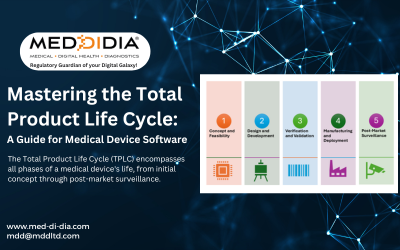
The Total Product Life Cycle (TPLC) encompasses all phases of a medical device's life, from initial concept through post-market surveillance.
14.1.2025
MDCG 2014-15 Guidance on Clinical Investigations

Provides guidance on the publication of clinical investigation reports and their summaries in the absence of the EUDAMED database.
2.12.2024
Celebrating Three Decades of Excellence: Platform94’s Inspiring Journey from GTC

As Platform94 celebrates an impressive 30 years of service, we are honoured to reflect on their remarkable transformation.
18.11.2024
Internationalising MedTech Innovation
Don’t miss this chance to gain critical insights from industry experts with decades of experience navigating the complexities of MedTech international
11.11.2024
Understanding the Certificate of Free Sale in the EU: A Comprehensive Guide

A Certificate of Free Sale (CFS) is an essential document for manufacturers & authorised representatives looking to export medical devices outside EU
23.10.2024