MDCG 2023-3 Guidance
24/2/2023
MDCG 2023-3 Guidance
Here is a brief summary of MDCG 2023-3, which is a document published in February 2023 by the Medical Device Coordination Group (MDCG) on vigilance terms and concepts as outlined in the Regulation (EU) 2017/745 on medical devices.
This document provides answers to frequently asked questions related to the vigilance requirements set out in the regulation, including the definitions of key terms such as "serious incident," "serious injury," and "serious public health threat." It also provides guidance on reporting requirements, risk assessment, and other aspects of the vigilance process.
Manufacturers and MDCG 2023-3
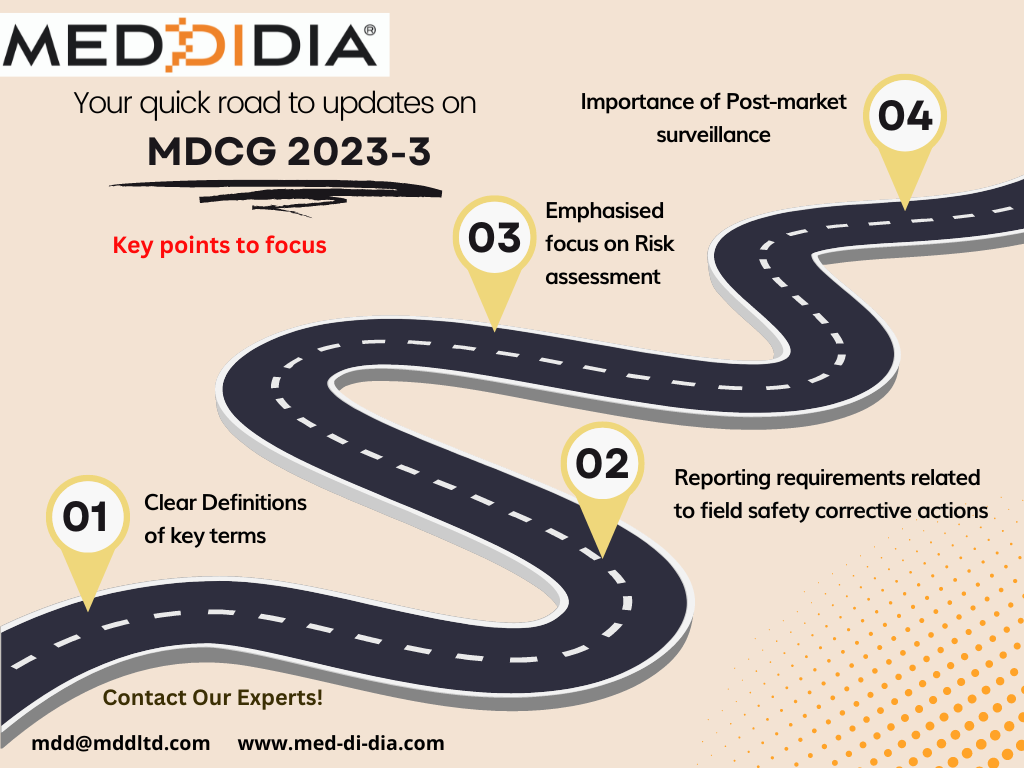
As a manufacturer you should be aware of the following key points from MDCG 2023-3:
- Definitions of key terms: The document provides clear definitions of key terms related to vigilance, such as "serious incident," "serious injury," and "serious public health threat." Manufacturers should ensure that they understand these definitions and use them consistently in their reporting and documentation.
- Reporting requirements: The document outlines the reporting requirements for incidents and field safety corrective actions (FSCAs), including the timelines for reporting and the information that should be included in the reports. Manufacturers should ensure that they have procedures in place to comply with these requirements.
- Risk assessment: The document emphasizes the importance of conducting a risk assessment for all incidents, and provides guidance on how to assess the severity and likelihood of harm, and how to determine whether an incident meets the criteria for reporting as a serious incident.
- Post-market surveillance: The document highlights the importance of post-market surveillance for monitoring the safety and performance of medical devices, and provides guidance on how to establish an effective post-market surveillance system.
MDCG 2023-3 aims to provide clarity and guidance on the vigilance requirements outlined in the regulation, to help ensure the safety and effectiveness of medical devices and protect public health.
Medical device manufacturers should carefully review MDCG 2023-3 to ensure that they understand the vigilance requirements outlined in the regulation, and to ensure that they have processes and procedures in place to comply with these requirements.
With the support of additional resources, our experts have summarised these points. If you are wondering how to ensure compliance with these and several other requirements, then feel free to contact our experts by sending an email – mdd@mddltd.com