Joint implementation and preparedness plan for Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR)
7/11/2022
A new legislative framework on medical devices Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) was adopted by the Council and the European Parliament in April 2017. This new framework sets high standards of quality and safety for medical devices and aims at ensuring the smooth functioning of the internal market. The IVDR had a date of application of 26 May 2022.
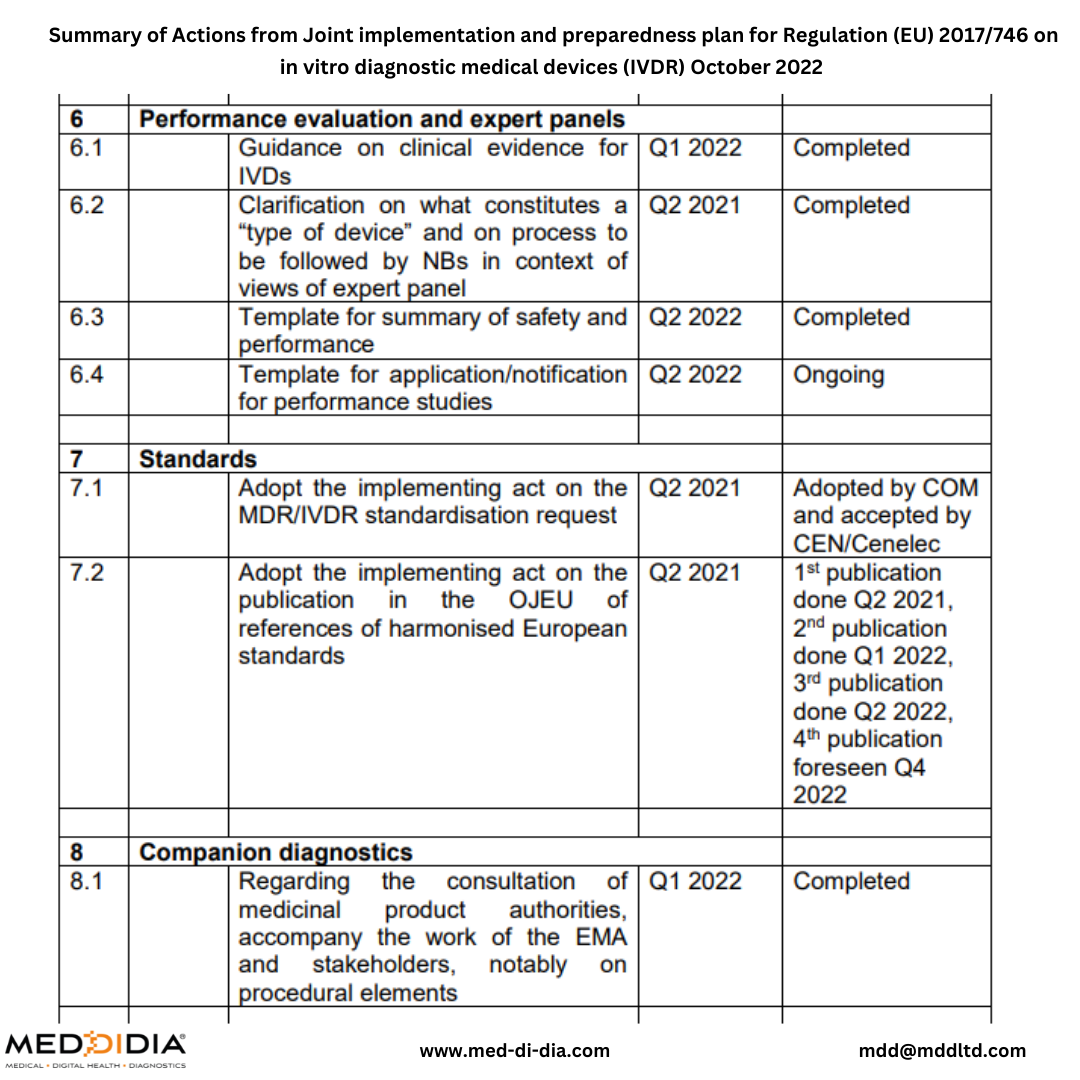
In October 2022, the European Commission published Joint implementation and preparedness plan for Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). This Joint Implementation Plan is the result of review by the MDCG including the relevant sub-groups, with input from stakeholders. In addition to setting the priorities, the Plan will serve as a living document to monitor their implementation.
Here is a Summary of Actions from Joint implementation and preparedness plan for Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) October 2022




Speak to our experts for all your Regulatory and Quality Management System requirements under Medical Devices, Diagnostics and Digital Health!
Drop us an email at mdd@mddltd.com







