EU MDR Timeline
7/3/2023
As of 08/12/2022 – EU MDR had the following timeline –
The new European Union Medical Device Regulation (EU MDR) was published in the Official Journal of the European Union on May 5th 2017 and “entered into force” 20 days later on 26 May 2017.
Readers should be aware that “entry into force” isn’t the same as being applicable. The existing Medical Device Directive (MDD) remains applicable, as there will be a transition from the current MDD to the new EU MDR. The end of the transition coming for the final few devices, potentially as late as 27th May 2025.
The details of the repeal of the current MDD are described in the new Article 122, with the transitional provisions being described in the new Article 120.
The key dates are as follows;
- Entry into force of the EU MDR - 26th May 2017
- Earliest date Notified Bodies may apply for designation according to the EU MDR - 26th Nov. 2017
- Earliest date EUDAMED can go live - 26th Mar 2021
- Date of application of the EU MDR - 26th May 2021
- Notified Body certificates issued under MDD designation become void (if not already expired) i.e. the last possible date for placing devices on the market according to the MDD - 26th May 2024
- Last possible date for putting devices into service according the MDD - 26th May 2025
On 09/12/2022

The European Commission proposed certain amendments to the Regulation where there was more emphasis on transitional dates of application. As always, the major focus remains on patient safety, followed by the availability of these Life-Saving Medical Devices at the right place at the right time!
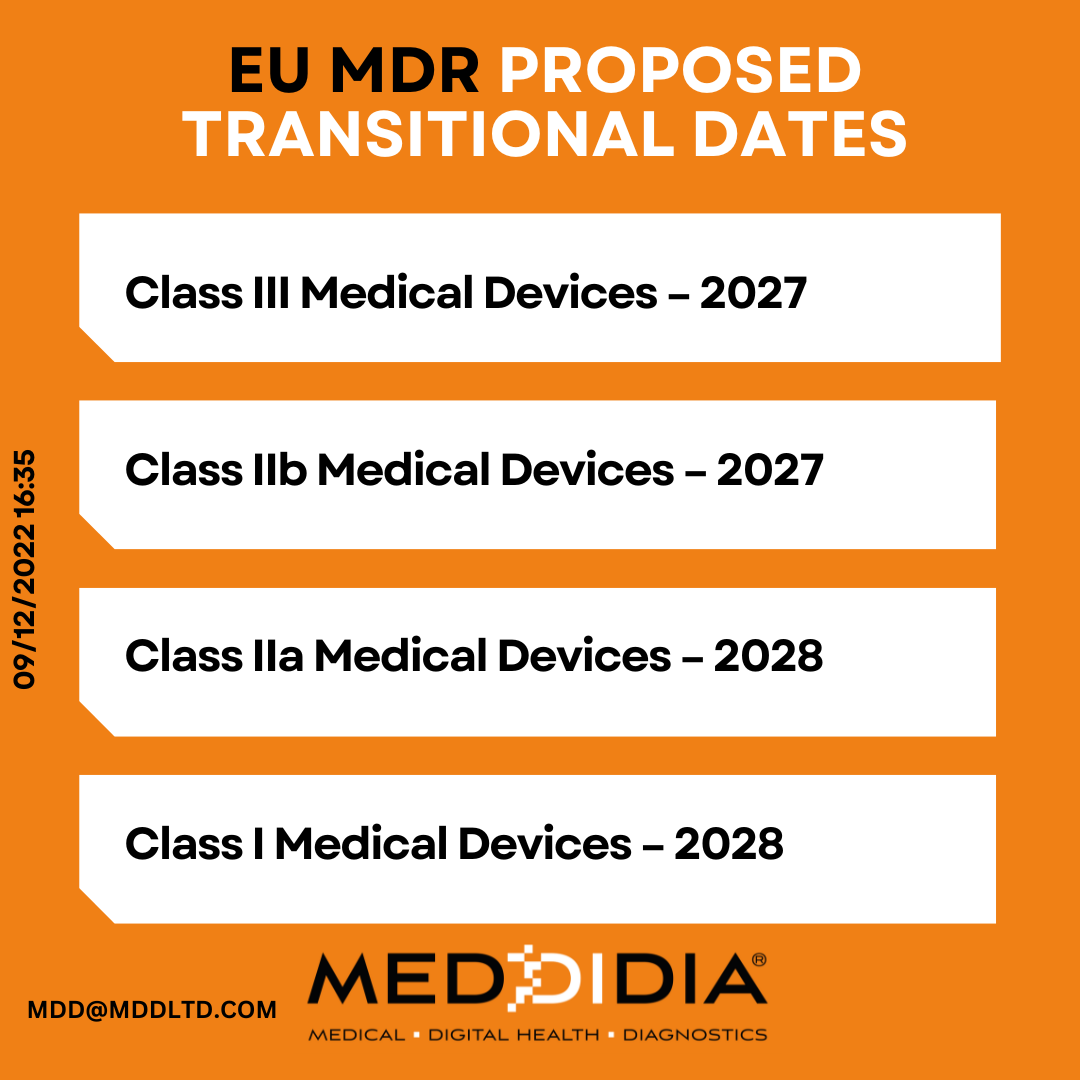
The proposed transitions are as follows:
- Class III Medical Devices – 2027
- Class IIb Medical Devices – 2027
- Class IIa Medical Devices – 2028
- Class I Medical Devices – 2028
while these proposed dates are yet to be signed as of 16:15 on Friday 09/12/2022, Manufacturers need to focus on the criteria under which these transitional dates can be adapted for their products.
In the upcoming days, the MDCG will publish a position paper with final updates, confirmations and any additional information.
What you need to do is
- Make sure your Quality Management Systems are in Place
- Ensure appropriate Transition plan from EU Medical Device Directives [EU MDD] to EU Medical Device Regulation [with possible extended transitions] are in Place.
- Contact Experts at Med-Di-Dia who can support you with completing your transition plans, technical files and documents.
- If you are yet to launch your medical device on the EU Market, speak to our experts, who can create a right Regulatory Roadmap for a smooth launch
We are here to be your Regulatory Risk Partners for Medical Devices, Diagnostics and Digital Health!
Drop us an email at mdd@mddltd.com
On 06/01/2023
Today, the Commission adopted a proposal to give more time to certify medical devices to mitigate the risk of shortages. The proposal introduces a longer transition period to adapt to new rules, as foreseen under the Medical Devices Regulation. The new deadlines depend on the medical devices' risk class and will ensure continued access to medical devices for patients. It will also allow medical devices placed on the market in accordance with the current legal framework and that are still available to remain on the market (i.e., no ‘sell-off' date).
This proposal does not change any of the current safety and performance requirements provided for in the Medical Devices Regulation. It only amends the transitional provisions to give more time for manufacturers to transition from the previously applicable rules to the new requirements of the Regulation. The length of the proposed extension of the transition periods depends on the type of device: higher risk devices such as pacemakers and hip implants will benefit from a shorter transition period (until December 2027) than medium and lower risk ones, such as syringes or reusable surgical instruments (until December 2028).
Key Updates:
- For medical devices covered by a certificate or a declaration of conformity issued before 26 May 2021, the transition period to the new rules is extended from 26 May 2024 to 31 December 2027 for higher risk devices and until 31 December 2028 for medium and lower risk devices. The extension will be subject to certain conditions, so that only devices that are safe and for which manufacturers have already taken steps to transition to the rules provided for by the Medical Devices Regulation will benefit from the additional time.
- The proposal introduces a transition period until 26 May 2026 also for class III implantable custom-made devices, giving their manufacturers more time to obtain certification by a notified body. Also in this case, the transition period is subject to the application of the manufacturer for a conformity assessment of devices of this type before 26 May 2024.
- To reflect the transition periods put forward by these amendments, the proposal extends the validity of certificates issued up until 26 May 2021, the day when the Medical Devices Regulation became applicable.
- The Commission also proposes to remove the ‘sell-off' date currently established in the Medical Devices Regulation and in the In Vitro Diagnostic Medical Devices Regulation. The ‘sell-off' date is the end date after which devices that have already been placed on the market, and remain available for purchase, should be withdrawn. Removing this ‘sell-off' date will ensure that safe and essential medical devices that are already on the market remain available to healthcare systems and to patients in need.
The proposal now needs to be adopted by the European Parliament and the Council through an accelerated co-decision procedure.
What should you do?
TAKE ADVANTAGE!
Our Experts are here to support you through this transitional dates. A robust Quality Management Systems can help you and your organisation in taken steps towards EU MDR compliance!
Commission full proposal: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_23
Factsheet: https://health.ec.europa.eu/system/files/2023-01/mdr_proposal_factsheet.pdf
Q&A: https://ec.europa.eu/commission/presscorner/detail/en/QANDA_23_24
Looking for support?
Drop an email to mdd@mddltd.com
Med-Di-Dia – Your Regulatory Risk Partners for Medical Devices, Diagnostics and Digital Health!


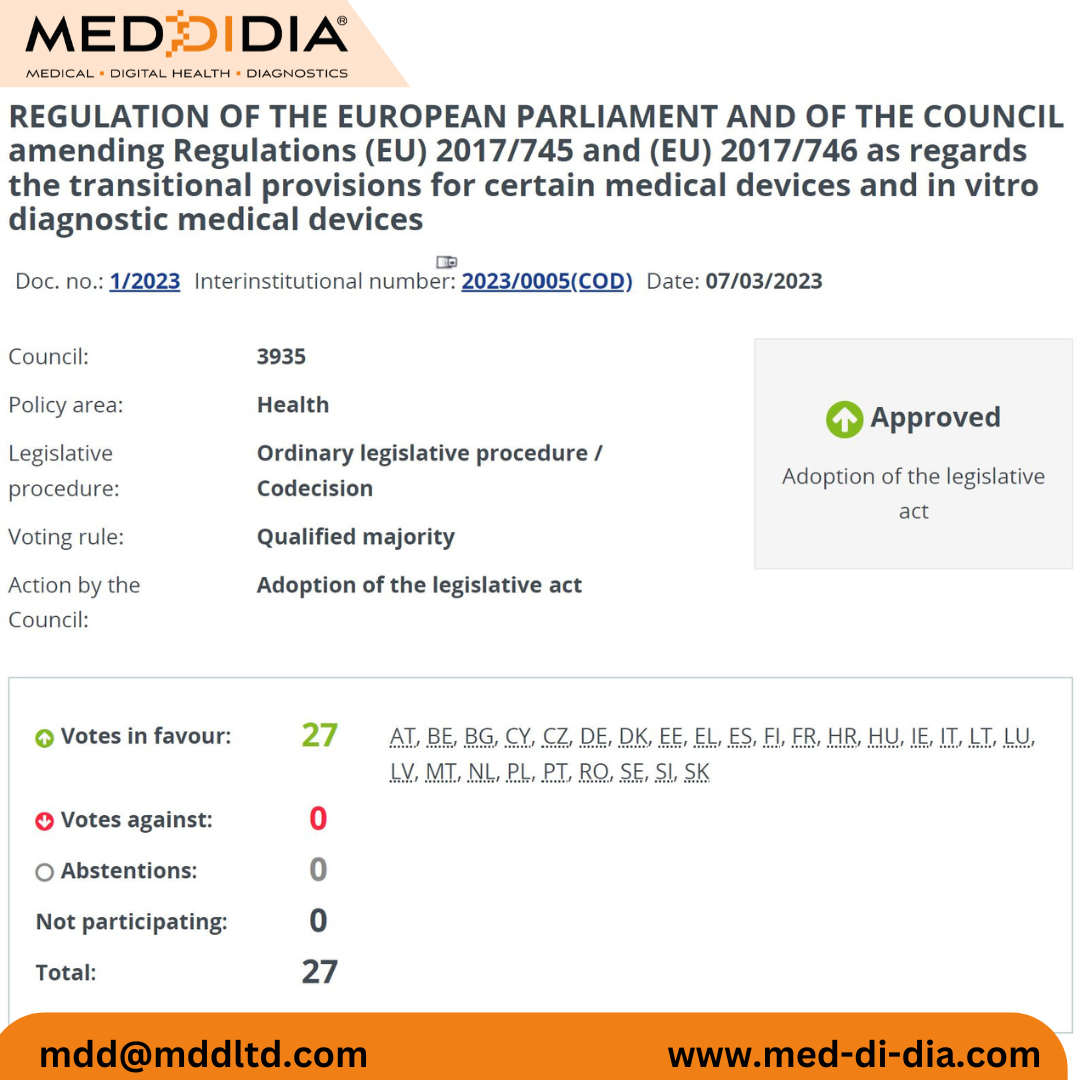
On 07th March 2023
The EU Commission Proposal focusing on the Ammendment of Transitional Deadlines has now been officially adopted and approved by all EU member states. Soon, the amendment will be available on Official Journal of the European Union [OJEU].
What do the key changes include?
- Extend the transitional period for higher-risk devices (class III and certain class IIb implantables) such as pacemakers to comply with EU MDR requirements until 31 December 2027, subject to certain conditions (including requirements for market surveillance, quality management systems, and engagement with notified bodies).
- Extend the transitional period for medium and lower-risk devices (other class IIb devices, class IIa, class Im, Is and Ir devices) such as syringes to comply with EU MDR requirements until 31 December 2028, subject to certain conditions (including requirements for market surveillance, quality management systems, and engagement with notified bodies).
- Extend the validity of certificates issued by notified bodies under Directive 90/385/EEC and 93/42/EEC that were valid on 26 May 2021. Subject to meeting specific conditions, this extension also applies to those certificates that expired before the amendments took effect.
What should a Medical Device Manufacturer do with the EU MDR Transitional Deadlines?
- Make sure your Quality Management Systems are in Place.
- Ensure appropriate Transition plan from EU Medical Device Directives [EU MDD] to EU Medical Device Regulation are in Place.
- Contact Experts at Med-Di-Dia who can support you with completing your transition plans, technical files and documents.
- If you are yet to launch your medical device on the EU Market, speak to our experts, who can create a right Regulatory Roadmap for a smooth launch
We are here to be your Regulatory Risk Partners for Medical Devices, Diagnostics and Digital Health!
Drop us an email at mdd@mddltd.com
What does the Extension Finally Mean?
In order to mitigate the risk of losing these critical medical devices on the EU market, the European Commission has now adopted an urgent, targeted legislative initiative to amend the MDR. In a recent press release, the European council highlighted that
Producers of medical devices will now have until 31 December 2027 for higher risk devices and until 31 December 2028 for medium and lower risk devices to meet the legal requirements.
The extension of the transition period will be granted under certain conditions. These ensure that only devices that are safe and for which manufacturers have already started the certification procedure will benefit from the additional time.
While the extension aims to relieve the supply chain, It's worth noting that this decision does not change the deadline for compliance with the MDR and IVDR, which remains May 26, 2024 and May 26, 2022, respectively.
In order to undertsand this better, our experts advise you to follow updates from - https://ec.europa.eu/commission/presscorner/detail/en/ip_23_23
- https://ec.europa.eu/commission/presscorner/detail/en/STATEMENT_23_1504
Or
Contact us on mdd@mddltd.com









