Notified bodies survey on certifications and applications
27/10/2022
On 26th October 2022 – the European Commission published their survey results on - Notified bodies survey on certifications and applications.
This survey was collected from 51 notified bodies in October 2022, where 47 Notified Bodies replied. The commission has considered data from the previous survey (April 2022) to cover up the missing replies.
This 18-page PowerPoint presentation from the European Commission highlights several factors related to MDR and IVDR applications. Some interesting trends could be noted from the results. One should keep in mind as of 25th October 2022 there are 34 Designated Notified bodies for EU MDR and 7 Designated Notified bodies for EU IVDR.
The European Commission is trying hard to cope with the exceeding demand for Notified Bodies and giving NANDO – EUDAMED – MDR – IVDR a smoother flow.
As a manufacturer, this is an important area to focus on –
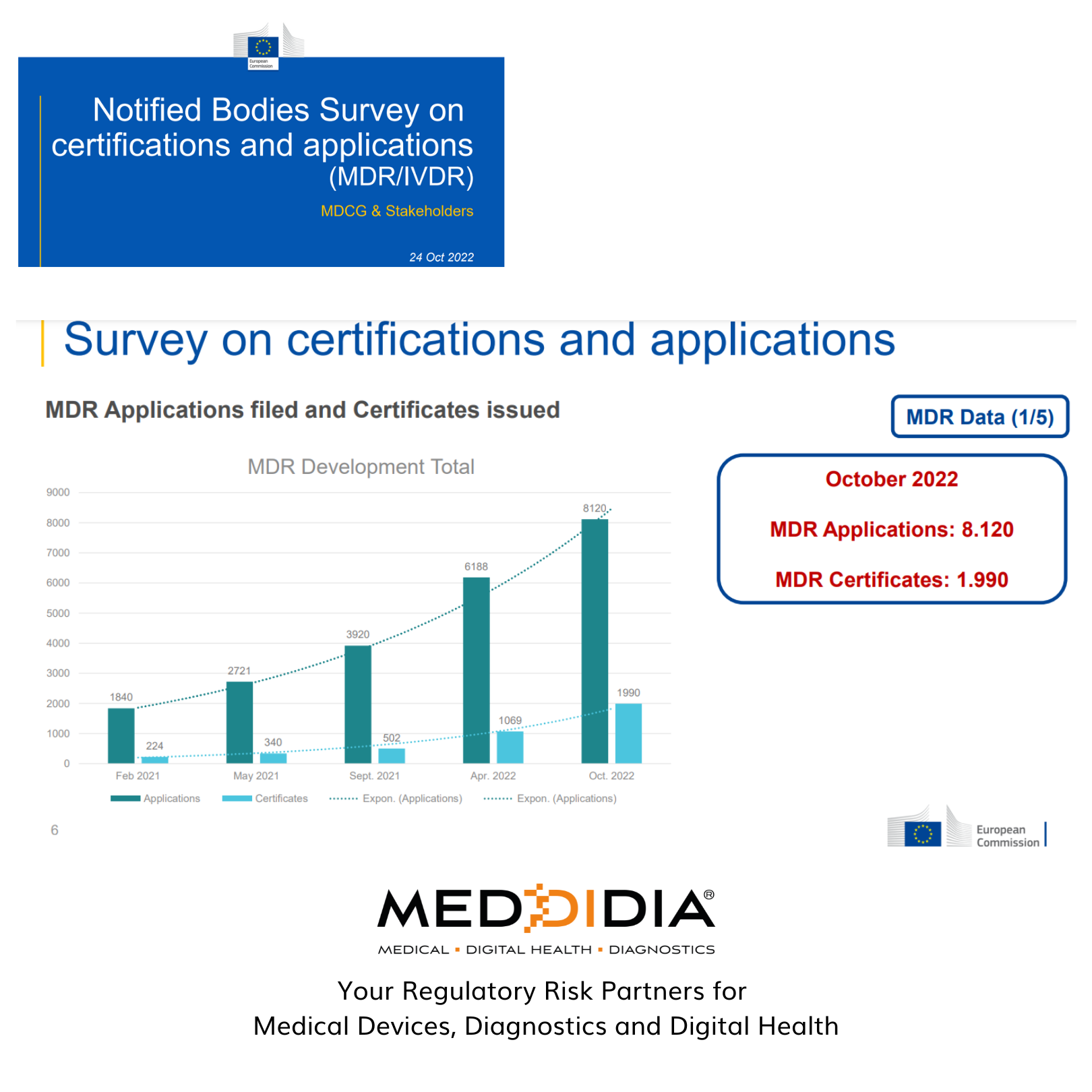
As of October 2022, all the responding notified bodies from the survey have a total of 8120 MDR applications, of which the notified bodies have issued only 1990 certificates.
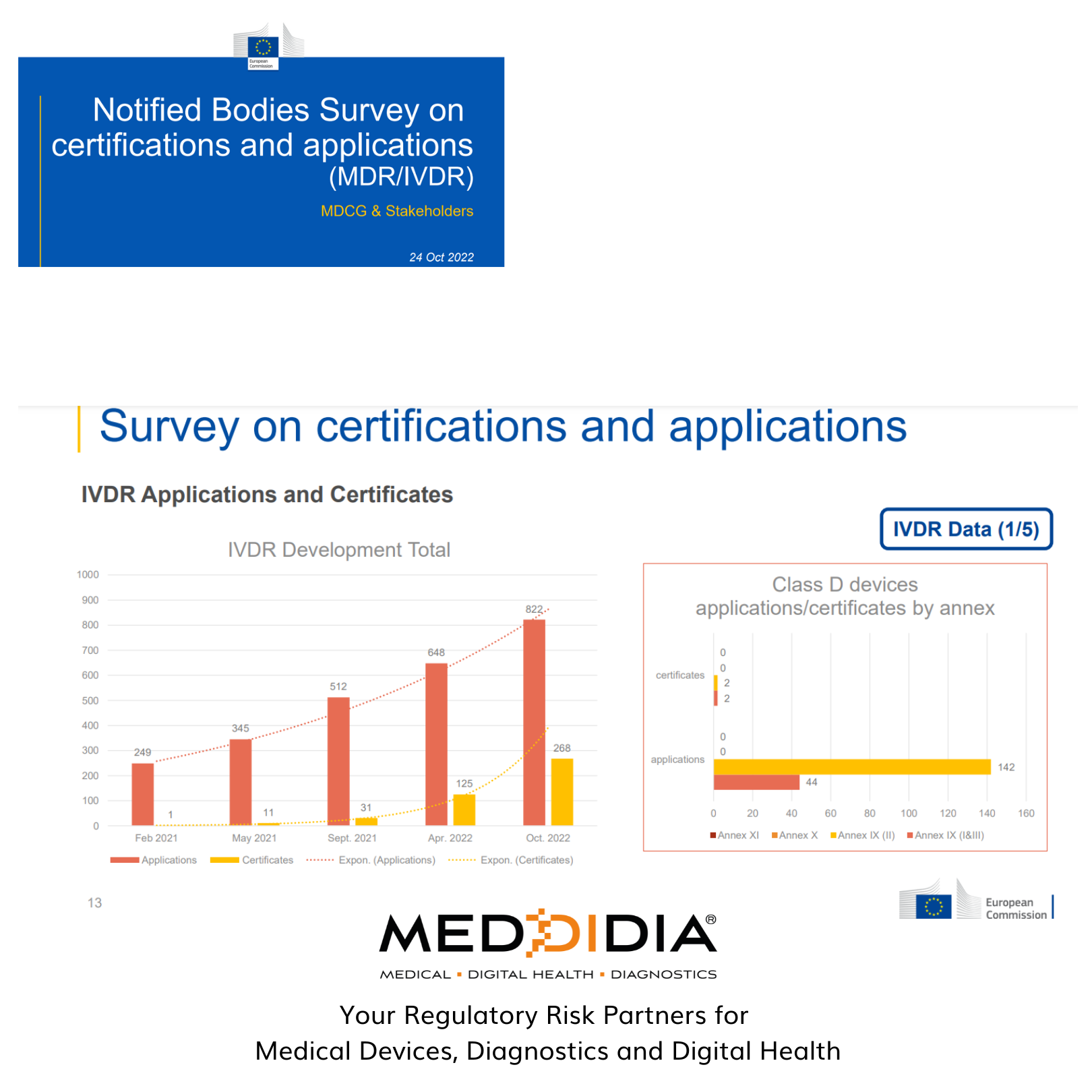
The same is the case with IVDR – where there are 822 IVDR Applications and only 268 certificates issued.
Since Feb 2021, there has been significant progress in the number of certificates issued under each case. Manufacturers still need to plan out the proper steps for getting their Medical Devices and Diagnostics certified and approved by the notified bodies.
One more worrying trend highlighted by our experts was the ‘Refusal Rate’ under EU MDR.
Based on the survey – one can find out that major certificates are refused because of
- Incomplete application
- Wrong Product Classification
- Submissions outside the scope of Notified Body’s designation
- Wrong conformity assessment procedure

In this dynamic world, one thing we know for sure is that ‘One Size doesn’t fit all!’
Our experts create bespoke solutions which will help you achieve the best possible roadmap for getting your Devices, Diagnostics certified under the EU MDR/ EU IVDR.
Mistakes can be avoided!
Get the right Person/Team!
Speak to Med-Di-Dia – Your Regulatory Risk Partner for Medical Devices, Diagnostics and Digital Health!
Connect with our experts by sending an email to mdd@mddltd.com







