MDCG advice for non EU/EEA SARS-CoV-2 infection IVD Manufacturers.
13/1/2022
MDCG 2022-1 (January 2022) - Addressees IVD manufacturers with the intended purpose to detect and/or quantify markers of SARS-CoV-2 infection who are based in countries outside the EU/EEA and who place or intend to place these devices on the EU market.
The scope includes
- Designation of an authorised representative
A manufacturer who does not have a registered place of business in an EU Member State and who intends to place devices on the EU market under his own name must designate an EU authorised representative
- Involvement of a notified body for self-tests under Directive 98/79/EC
Under Directive 98/79/EC, SARS-CoV-2 IVDs intended to be used by professionals do not need assessment by a notified body. In contrast, for SARS-CoV-2 self-tests, i.e. those intended for lay users, the manufacturer must lodge an application with a notified body
- Translations of the instructions for use and the label
Manufacturers must ensure good quality of translations of the instructions for use and of the text elements of the label into all languages required by the Member States where the device is made available.
- Guidance on performance evaluation of SARS-CoV-2 IVDs The Medical Device Coordination Group document MDCG 2021-21 provides guidance on the performance evaluation of different types of SARS-CoV-2 IVDs, including SARS-CoV-2 self-tests: click here - This document is relevant for devices being placed on the market under Directive 98/79/EC and under Regulation (EU) 2017/746. [See bottom of the page]
- Transition to Regulation (EU) 2017/746
Under Regulation (EU) 2017/746, all SARS-CoV-2 IVDs are subject to conformity assessment involving a notified body. Manufacturers that wish to place devices on the market in accordance with Regulation (EU) 2017/746, whether based in the EU/EEA or in 3rd countries, that have not yet submitted an application for conformity assessment to a notified body are strongly encouraged to liaise with designated notified bodies as soon as possible
Do you want to know what it means for your #MedicalDevice?
Send us an email at mdd@mddltd.com and our team of experts will assist you.
We are here to be your Regulatory Risk Partners for Medical Devices, Diagnostics and Digital Health!
Read The Detailed Address by clicking here
On 15th Febreuary 2022,
an updated version of MDCG 2021-21 Guidance on performance evaluation of SARS-CoV-2 in vitro diagnostic medical devices was published.
This update talks about the revision of the following:
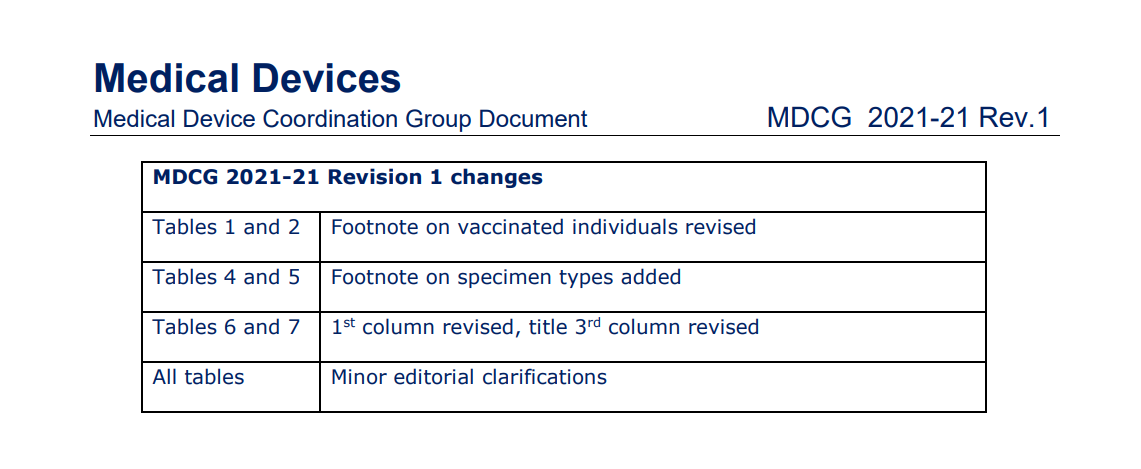
Detailed Publication can be found here
Are you Looking for more clarification?
Contact our experts by sending an email at mdd@mddltd.com
Med-Di-Dia - Your Regulatory Risk Partners for Medical Devices, Diagnostics and Digital Health!







