Latest Guidance on UDI by USFDA
8/7/2021
On 7th July 2021, USFDA released Guidance for Unique Device Identification System: Form and Content of the Unique Device Identifier (UDI).
This Guidance describes the two forms of a UDI, clarifies the UDI content, and addresses the use of data delimiters that identify specific data elements within the UDI. The Guidance also addresses the recommended order of the data in the easily readable plain-text form of a UDI carrier.
This Guidance does not apply to universal product codes (UPCs). For Class I devices, a UPC may serve as the UDI to meet the requirements of 21 CFR 801.20 (21 CFR 801.40(d)). However, a Class I device labeller may choose to use a UDI rather than or in addition to a UPC (see 21 CFR 801.35). For more information on UPCs, labellers should contact an issuer of UPCs. Labelers should have proper controls over UPC assignment and use to advance the goals of the UDI system.
As we all know, “Unique device identifier” is defined as “an identifier that adequately identifies a device through its distribution and use by meeting the requirements of [21 CFR 830.20]” (21 CFR 801.3). A UDI is composed of a
- Device Identifier (DI)
“Device identifier” is defined as “a mandatory, fixed portion of a UDI that identifies the specific version or model of a device and the labeller of that device”(21 CFR 801.3).
- Production identifier (PI)
“Production identifier” is defined at 21 CFR 801.3 as “a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of the device:
- The lot or batch within which a device was manufactured.
- The serial number of a specific device
- The expiration date of a specific device
- The date a specific device was manufactured
- For an HCT/P regulated as a device, the distinct identification code required by 21 CFR 1271.290(c) of this chapter.”
Along with defining UDI and its components, the Guidance underlines other key terms:
Forms of UDI - The UDI Rule specifies that the UDI on the device label and device packages must be presented in both easily readable plain-text and AIDC technology forms (21 CFR 801.40(a)).
- Easily Readable
The easily readable plain-text form of the UDI must include the DI and, if applicable, PI. It is also important for the easily readable plain-text form of the UDI to include any data delimiter(s) from the DI and PI.
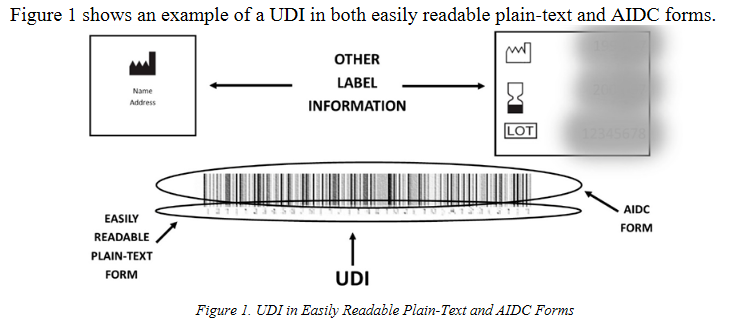
- AIDC
AIDC is defined at 21 CFR 801.3 as any technology that conveys the UDI or the DI portion of a UDI of a device in a form that can be entered into an electronic patient record or other computer systems via an automated process. Therefore, while the UDI Rule does not require the use of a specific form of AIDC or a specific AIDC technology to convey the UDI, the AIDC form of the UDI must be in a format that can be read by a bar code scanner or other AIDC technology (21 CFR 801.3 and 801.40(a)).
Contents of the UDI –
Under 21 CFR 801.40(b), the easily readable plain-text and AIDC forms of a UDI must include:
(1) a device identifier segment; and
(2) a production identifier segment that conveys one or more of the identifiers enumerated in the definition of a “production identifier” at 21 CFR 801.3 when included on the device label.
The device identifier segment and the production identifier segment cannot include non-UDI elements or data delimiters for non-UDI elements.
Special instructions for Stand-alone software:
There are different labelling requirements for stand-alone software depending on whether or not it is distributed in packaged form (21 CFR 801.50). For stand-alone software that is not distributed in packaged form, the UDI labelling requirements are met if the UDI is provided through either or both of the following and the version number is conveyed in its PI: an easily readable plain-text statement displayed whenever the software is started; or· an easily readable plain-text statement displayed through a menu command (e.g., an “about” command) (21 CFR 801.50(a)).
The Guidance brings in additional load on the regulatory front. Non-compliance to these guidelines can result in severe regulatory mishaps causing lot/product recall.
Save time, Connect with our experts now!
Call: +353 (0)91-704804
Email: mdd@mddltd.com
Access the guidance here







